Substances That Exist as Gases
Topic Description
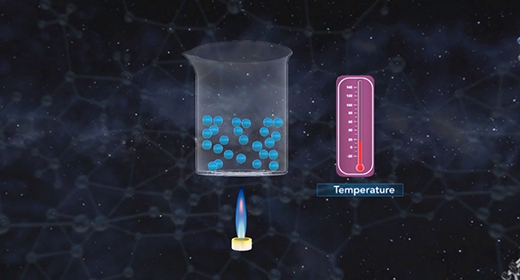
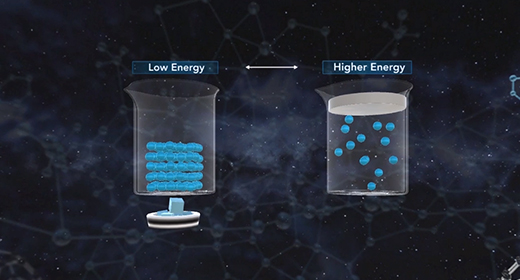
Upon completion of this module, you should be able to illustrate the concept of the kinetic theory of matter and know the substances that exist as gases at room temperature. The kinetic theory of matter provides a basic overview of why matter exists in three different states. The kinetic theory of matter states: The matter is made up of constantly moving particles. All particles have energy, but the energy varies depending on whether the substance is a solid, liquid, or gas; solid particles have the least amount of kinetic energy, and gas particles have the most. The temperature of a substance is the measure of the average kinetic energy of the particles. A change in phase may occur when the energy of the particle changes. There are spaces between the particles of matter.

The topic "Substances That Exist as Gases" is available as part of this package. Subscribe this package to get access to this topic.