Strength of Acids and Bases
Topic Description
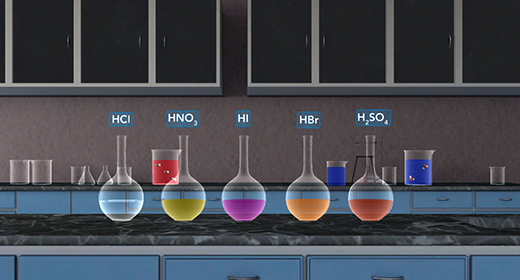
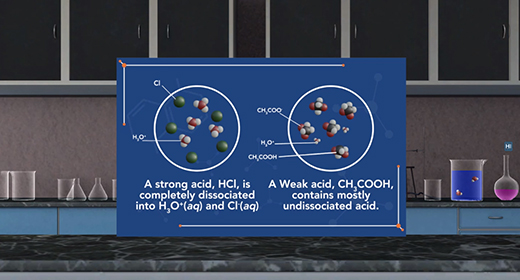
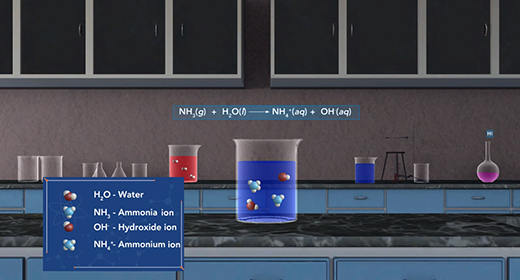
Upon completion of this module, you should be able to explain the differences between a strong acid and a weak acid and explain the differences between a strong base and a weak base. When an acid dissolves in water, a proton transfer from the acid to water to form a hydronium ion. This process is called dissociation. We can rank the strengths of a list of acids by the extent to which they ionize in aqueous solution. When a strong acid is added to water, 100 percent of the acid dissociates into ions. For example, a strong acid, hydrochloric acid is completely dissociated into hydronium and chloride ions in water.

The topic "Strength of Acids and Bases" is available as part of this package. Subscribe this package to get access to this topic.