Ionization Energy
Topic Description
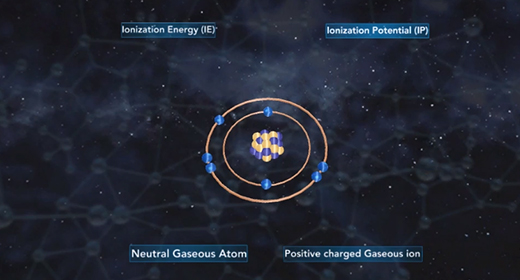
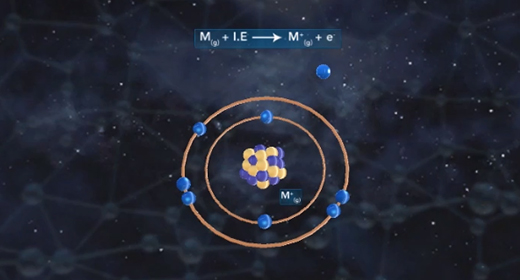
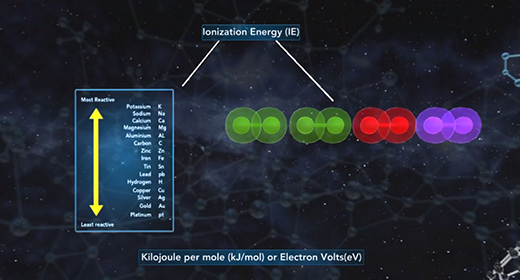
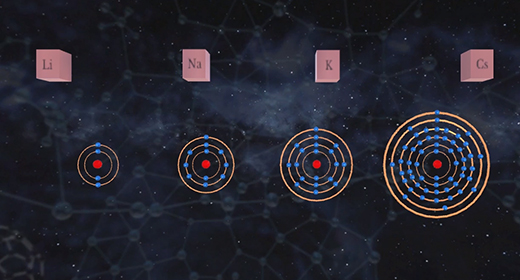
Upon completion of this module, you should be able to understand the basic concept of ionization energy and understand the trend for ionization energy in the Periodic Table. Ionization energy (IE) is the minimum energy required to remove an electron from a neutral gaseous atom in the ground state and convert it into a positively charged gaseous ion. Ionization energy is also known as ionization potential (IP) which is written as Ho. In the example below, M(g) is a neutral gaseous atom in the ground state, I. E. is ionization energy, M+(g) is a positively charged gaseous atom, and e- is the electron that has been removed.

The topic "Ionization Energy" is available as part of this package. Subscribe this package to get access to this topic.