Heat of Solution
Topic Description
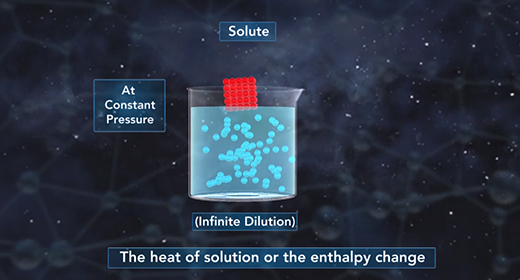
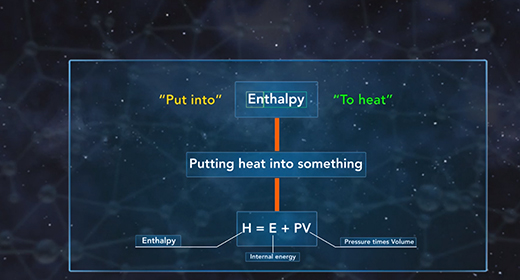
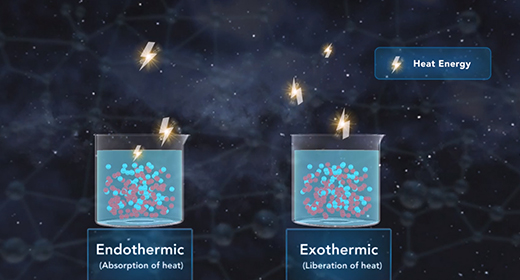
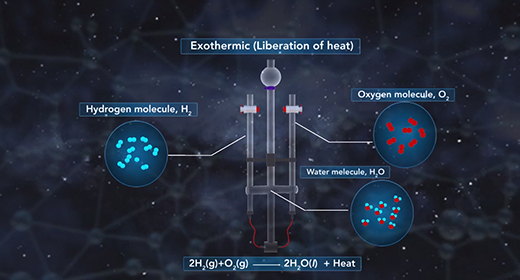
Upon completion of this module, you should be able to understand the heat of the solution and know the difference between endothermic and exothermic reactions. The heat of solution is the enthalpy change associated with the dissolution of a solute in a solvent at constant pressure resulting in infinite dilution. The heat of solution, like all enthalpy changes, is expressed in KJ/mol for a reaction taking place at standard conditions. The heat of solution is also referred to as enthalpy of solution or enthalpy of dissolution.

The topic "Heat of Solution" is available as part of this package. Subscribe this package to get access to this topic.