Energy Changes in Chemical Reactions
Topic Description
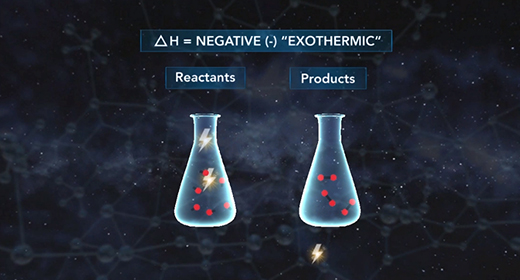
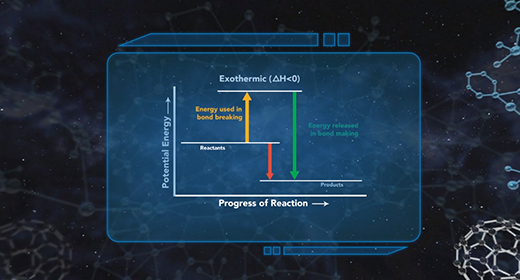
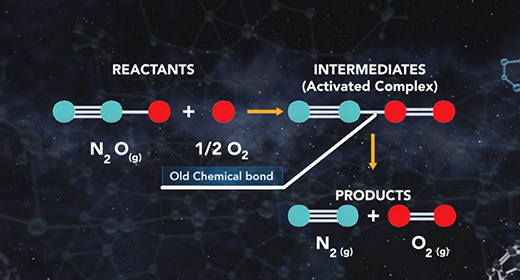
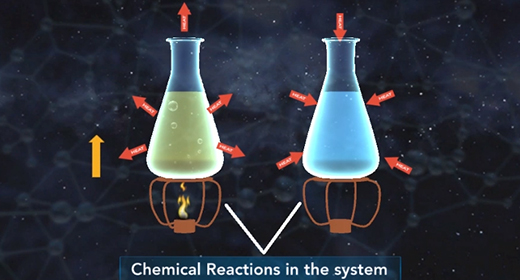
Upon completion of this module, you should be able to understand the basics of energy changes in chemical reactions and distinguish between exothermic and endothermic reactions. All chemical reactions involve energy changes. In some reactions, it is possible to observe these energy changes as either an increase or a decrease in the overall energy of the system. The study of energy changes (particularly heat) in chemical reactions is known as chemical thermodynamics or thermochemistry. Energy is absorbed when chemical bonds are broken, and energy is released when chemical bonds form.

The topic "Energy Changes in Chemical Reactions" is available as part of this package. Subscribe this package to get access to this topic.