Dual Nature of Electron
Topic Description
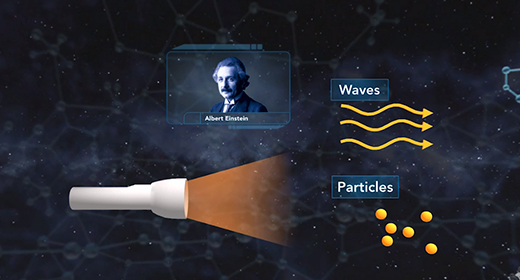
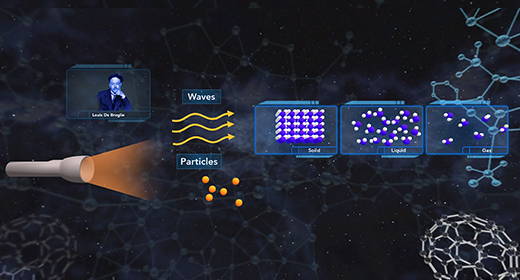
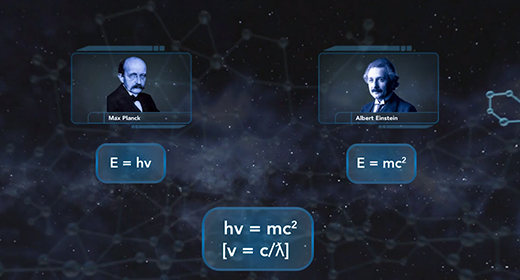
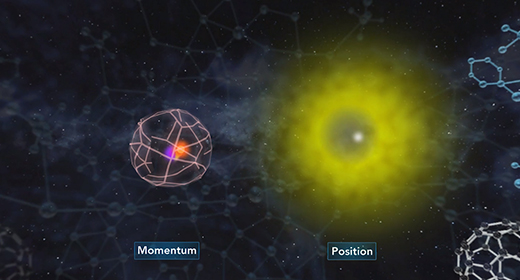
At the end of the video, you will be able to explain the dual nature of the electron and the derivation of the de Broglie equation and Heisenberg uncertainty principle. In 1905, Albert Einstein suggested that light behaves like a beam of particles as well as a wave. In 1924, French physicist Louis De Broglie suggested that if light has the characteristics of both a particle and a wave, the matter that is in motion would also behave as both a particle and a wave. The wavelength of matter in motion could be calculated using the following equation ? = h / mv.

The topic "Dual Nature of Electron" is available as part of this package. Subscribe this package to get access to this topic.