Diprotic and Polyprotic Acids
Topic Description
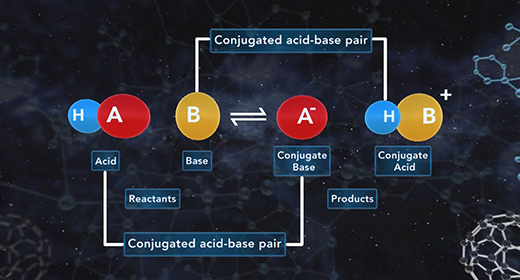
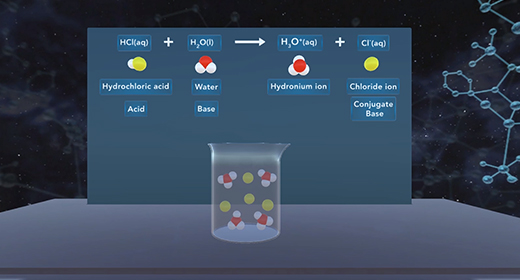
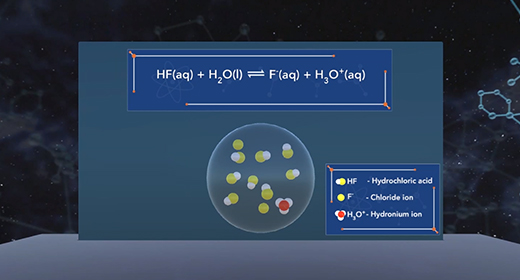
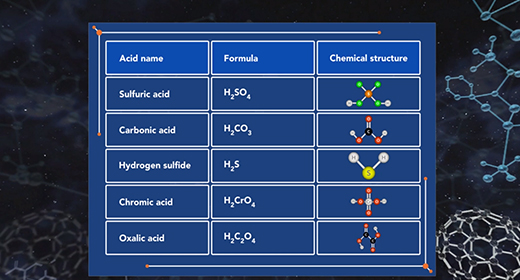
Upon completion of this module, you should be able to understand the difference between monoprotic, diprotic, and polyprotic acids and understand the process of dissociation of diprotic and polyprotic acids using acid-dissociation constants. Monoprotic acids Brønsted-Lowry acids donate hydrogen ion, and Brønsted-Lowry bases accept a hydrogen ion. For example, when hydrochloric acid (HCl) is added to water (H2O), the HCl donates a proton to water forming Hydronium ion (H3O+). HCl is an acid because it donates a proton (H+), and H2O is a base because it accepts the proton. A diprotic acid is an acid that can donate two protons or hydrogen ions per molecule to an aqueous solution. Acids that can donate two hydrogen ions are called diprotic acids. A polyprotic acid is an acid that can donate more than two protons or hydrogen atom per molecule to an aqueous solution.

The topic "Diprotic and Polyprotic Acids" is available as part of this package. Subscribe this package to get access to this topic.