Colloids
Topic Description
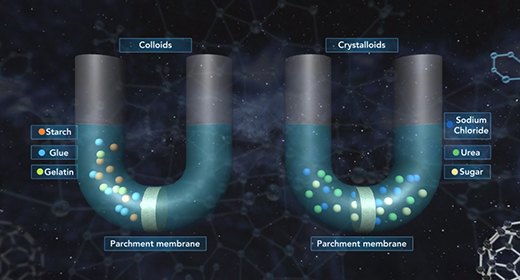
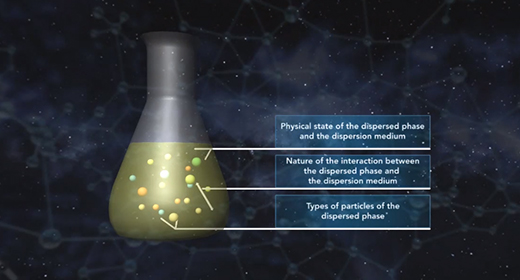
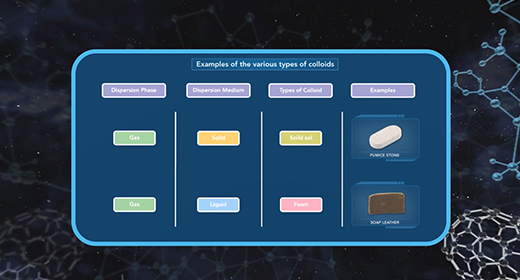
Upon completion of this module, you should be able to understand the classification of colloids and differentiate between various types of colloids. In 1861 Thomas Graham observed that certain solutes such as starch, glue, and gelatin could not pass through a parchment membrane while other solutes such as sodium chloride, urea, and sugar could easily do so. Graham called the former solutes colloids and the latter solutes crystalloids.

The topic "Colloids" is available as part of this package. Subscribe this package to get access to this topic.