Acid-Base Titrations
Topic Description
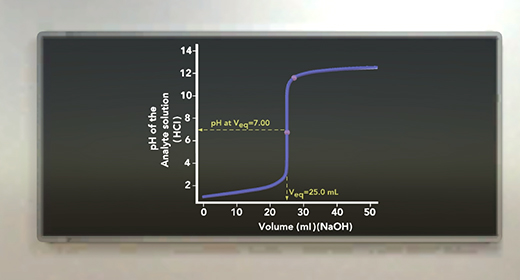
Upon completion of this module, you should be able to understand a titration set up, explain the role of indicators in titrations, and complete acid-base titration calculations. A titration is a reaction between a solution with a known concentration (titrant) with another solution with an unknown concentration (analyte) until the reaction reaches neutralization. Neutralization is a point that can be detected by a color change. The color change is achieved with a drop or two of a suitable indicator usually added to the solution of unknown concentration.

The topic "Acid-Base Titrations" is available as part of this package. Subscribe this package to get access to this topic.